Abstract
The dramatic responses induced by CAR-T cell therapy in the treatment of hematological malignancies has led to significant fanfare and the hope of durable cures. There has been considerable excitement in the myeloma field with the explosion of BCMA-targeting CAR-T cells, culminating with the recent approval of idecabtagene vicleucel. Unfortunately, this excitement has been tempered by the high rate of relapse either due to target antigen loss or through antigen-independent mechanisms. Meanwhile, the development of additional CAR-T cell therapies that may be used as an alternative to, or in conjunction with, BCMA-targeting CAR-T cells has been hampered by difficulties associated with identifying targets. We previously described semaphorin 4A (SEMA4A) as an effective and safe antibody-drug conjugate target in myeloma. Here, we show that SEMA4A is also a strong candidate for targeting by CAR-T cells.
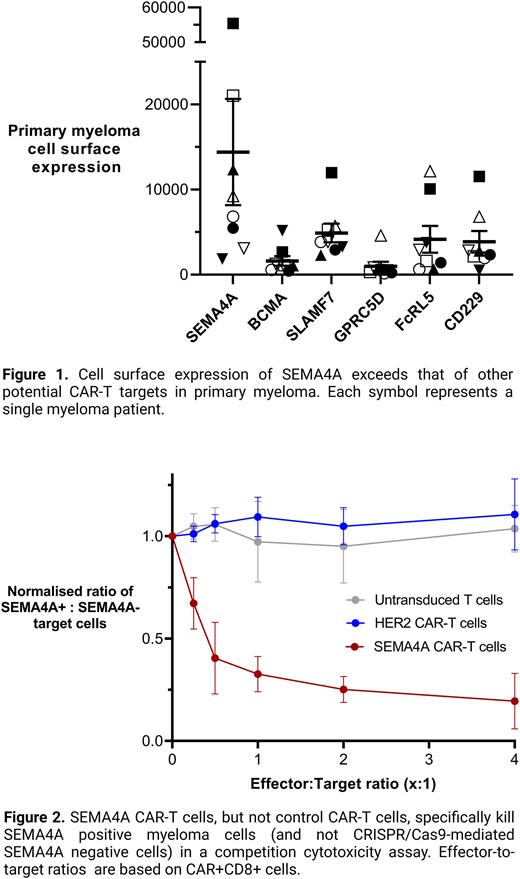
Cell surface expression of SEMA4A in primary myeloma cells, as determined by plasma membrane fractionation and mass spectrometry exceeds that of BCMA and other mooted CAR-T targets (Fig. 1). Importantly, flow cytometry not only confirms this, but also demonstrates that expression of SEMA4A is more ubiquitous than BCMA across cell populations of individual patients, and that SEMA4A is expressed in myeloma cells that are BCMA-negative. Given our previous data demonstrating that SEMA4A has limited off-tumour expression and that its expression is required for myeloma cell survival, we decided to generate a SEMA4A-targeting CAR-T cell for treatment of myeloma.
However, we first had to address two potential limitations. Firstly, whilst SEMA4A expression on resting T-cells is minimal and specific to a subset of CD8+ T-cells, its expression is upregulated post-activation, meaning that fratricide of T-cells by a SEMA4A-targeting CAR-T cell could present difficulties. To address whether abrogation of SEMA4A expression was a viable engineering strategy for a CAR-T vector, we developed an shRNA that specifically downregulated SEMA4A in activated T-cells. Importantly, this had minimal impact on measured growth or activity of the T-cells.
The second challenge is moderate off-tumour expression of SEMA4A on monocytes. Although this expression is lower than that on myeloma cells, we anticipate that persistent monocytopenia in the face of long-lived SEMA4A-targeting CAR-T cells would be a limitation of therapy. We have identified dozens of cell surface proteins that are expressed on monocytes, but not on myeloma cells. This presents the opportunity to use a NOT-gate approach to prevent targeting of monocytes. As a proof of principle, we coupled a HER2 scFv to the PD1 signalling machinery as part of a SEMA4A-targeting CAR-T vector. This prevented killing of myeloma cells ectopically expressing HER2, but not wild-type myeloma cells, thus demonstrating that our NOT-gate prevents unwanted cell killing but does not interfere with killing of SEMA4A-expressing target cells. This vector can easily be modified to express an scFv against any of the monocyte-specific cell surface proteins and we are currently selecting the optimum choice of NOT-gate for the final construct.
Having overcome the major hurdles for targeting SEMA4A by CAR-T cells, we generated the first SEMA4A CAR-T cell. This second-generation CAR construct contains a humanised scFv targeting SEMA4A followed by a CD8 hinge region, a CD28 transmembrane domain, a CD28 costimulatory domain and the CD3z signalling domain. We transformed donor T-cells with this construct and tested its ability to target specifically SEMA4A-expressing myeloma cells in a competition assay. SEMA4A-negative cells generated by CRISPR/Cas9 targeting were co-cultured with wild-type myeloma cells and SEMA4A-targeting or control CAR-T cells. This demonstrated potent and selective killing of only the wild-type myeloma cells by the SEMA4A CAR-T cells, but not by control CAR-T cells (Fig. 2).
In summary, we have demonstrated that SEMA4A can be effectively and safely targeted by CAR-T cells in myeloma, provided suitable engineering modifications are made to the vector. Ongoing work is focused on identifying further refinements to optimize the vector, prior to in vivo testing of SEMA4A CAR-T cells.
Disclosures
Walker:Abbvie: Consultancy. Chapman:Sanofi: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.